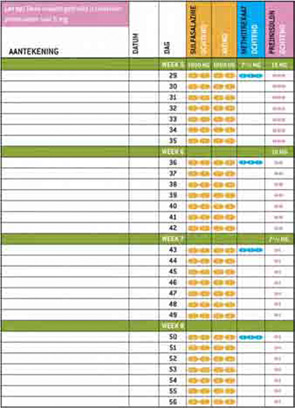
To further improve patient comfort while using COBRA therapy, the patient information material also contained a written interview in which a fictional early RA patient explained her feelings during diagnosis and the start of COBRA therapy. The content of this interview was also derived from the focus group discussions with patients and was used in the booklet to give patients a positive boost when starting this therapy. We provided a group of rheumatologists and arthritis nurses with this implementation material and asked them to prescribe COBRA therapy to their patients using the supplied material. All parties involved—rheumatologists, patients, and nurses—evaluated the material and gave positive responses. The website, which features the patient information booklet as well as all the scientific research around COBRA therapy presented at congresses and in articles is currently online at www.cobratherapy.nl. With this pilot study, the material is ready to be implemented in clinical practices around the world.
The Future of COBRA Therapy
Our findings and clinical experience indicate that COBRA therapy is effective, safe, and—with the proper education materials—easy to use in clinical practice by patients as well as rheumatologists and arthritis nurses. Moreover, worldwide implementation of COBRA therapy as a first choice for newly diagnosed RA patients with an aggressive arthritis makes sense, not only from a medical perspective, but perhaps also from an economic one and a patient’s point of view. Because COBRA therapy is a combination of inexpensive, generic drugs, it is a highly cost-effective treatment of early RA. Patients would benefit from implementation of COBRA therapy because this therapy gives them a better prognosis than DMARD monotherapy.
We suggest that rheumatologists around the world strongly consider COBRA therapy for patients with active RA on first presentation. In case of failure to respond, patients can rapidly switch to a biological in combination with methotrexate, as the conditions set by some national authorities to have failed on at least two DMARDs have been met. This strategy ensures fast, effective, and cost-effective treatment for early active RA patients, increasing their chances to regain their quality of life and prevent long-term damage to their joints.
COBRA therapy is probably most often used in the Netherlands, where the COBRA trial was originally conducted (in cooperation with Leuven, Belgium). The Dutch website, which has been online for a year, is frequently visited. More recently, we learned that COBRA therapy has been implemented at the Bristol University Royal Infirmary in the U.K., where a group of the most severely affected early RA patients receive COBRA therapy as standard care.