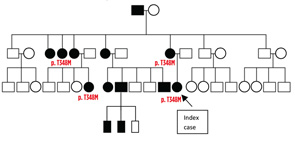
When I (PJH) first saw the patient, it was clear she had hearing difficulties, and her mother had hearing aids in both ears. The patient also had an urticarial rash and red eyes. Genetic studies discovered the T348M pathogenic variant on one allele of the NLRP3 gene in the patient, her mother and other family members. Treatment with canakinumab led to a dramatic decrease in all symptoms except headaches, and she entered a rapid growth phase, including pubertal development. There was normalization of the acute-phase reactants. Her hearing loss did not progress.
Discussion
These cases demonstrate the difficulty and delay in recognizing and diagnosing the rare autosomal-dominant cryopyrin-associated periodic syndromes (CAPS). CAPS is an autoinflammatory syndrome that consists of three classic phenotypes, described clinically over a period of 70 years, although only recently appreciated to involve similar mechanisms.
The first and mildest phenotype, described in 1940 by Kile and Rusk, was originally termed familial cold urticaria and is now called familial cold autoinflammatory syndrome (FCAS).1
The second clinical phenotype was described in 1962 and is named after the authors, Muckle-Wells syndrome (MWS).2
The third and most severe phenotype, described in the 1970s and early 1980s, is called neonatal-onset multisystem inflammatory disease (NOMID) in North America and chronic infantile neurological, cutaneous and articular syndrome (CINCA) in Europe.3
Familial occurrences of FCAS and MWS were frequently observed, and in 2001, Hal Hoffman, MD, professor of pediatrics and medicine, University of California, San Diego, and his group discovered the genetic etiology of the disease as gain of function mutations in the cold-induced autoinflammatory syndrome 1 (CIAS1) gene on the long arm of chromosome 1, encoding the cryopyrin protein.4 Later, the name of the gene was changed to the nucleotide-binding oligomerization domain-like receptor family, pyrin domain containing 3 (NLRP3) gene, based on the structure and function of the protein (also known as NLRP3). Due to the severity of the NOMID/CINCA phenotype, only rare familial cases were described, but a number of clinical features common to NOMID/CINCA and to FCAS and MWS, specifically rash, eye inflammation and hearing loss, led to the discovery by Ann-Marie Prieur, MD, from the Unit of Pediatric Immunology-Hematology and Rheumatology at the Hôpital Necker-Enfants Malades in Paris, France, and colleagues that the genetic etiology of NOMID/CINCA is identical to FCAS and MWS.5,6

