Environmental Exposure
As mentioned above, the description of RA has only been recorded since the industrial revolution, supporting a role for the environment. This is further supported by the observation that monozygotic twins are concordant for RA in only about 15% of cases. Smoking was long known to be associated with RA and the presence of rheumatoid factor.10 A number of studies have shown that smoking is strongly associated with ACPA-positive RA.11 The mechanism by which smoking may interact with the SE to produce disease was recently clarified by the observation that smoking was associated with the presence of citrullinated proteins inside alveolar macrophages obtained by lavage of normal smokers.11 Further, the bronchus-associated lymphoid tissue identified in some patients with pulmonary complications of RA produced ACPAs, suggesting that the lung might be the original site for producing ACPAs in some patients.12 Other studies demonstrate that periodontal disease results in the presence of citrullinated proteins locally. These observations identify mechanisms by which environmental exposure may produce citrullinated proteins.
Another environmental element that may contribute to the pathogenesis of RA is the gut microbiome. Studies demonstrate that a variety of forms of experimental arthritis do not develop if the mice are raised in a germ-free environment in which they lack microbial colonization of the intestine. Further, colonization with even a single strain of intestinal bacteria promotes disease expression. A recent article in The Rheumatologist by Drs. Scher and Abramson eloquently reviewed this topic, describing how imbalance of the intestinal microbiota results in an increase of disease-associated lymphocytes including TH1, TH2, and TH17 cells and a reduction of suppressive T regulatory cells, which may promote disease.13 These observations from experimental animals suggests that the intestinal microbiome has the potential to set the immunologic rheostat, perhaps in concert with the SE and other disease-associated SNPs, to promote the induction of the immunologic response that ultimately results in RA.
Preclinical Immune Response
A number of studies demonstrate that rheumatoid factor and ACPAs may be present long before the onset of RA, while a recent study demonstrates that epitope spreading occurs for years prior to the onset of disease and that citrullinated epitopes may be present on multiple autoantigens.5,14 The T cell–dependent induction of ACPAs is mediated by the presentation of citrullinated epitopes bound to cell-surface SEs to antigen-specific T cells, which then provide help to B cells to promote the production of the antibodies.2 Since the autoantibodies may be detected long before the onset of the disease, these observations support the role of the innate immune system as antigen-presenting cells, and T and B lymphocytes in the production of autoantibodies prior to the onset of the disease. Further supporting the role of the innate immune system, IL-6 and soluble TNF receptor II, a proxy for TNF-α, were elevated in plasma samples taken from patients up to 12 years prior to the onset of RA.15 Together, these observations demonstrate that both the innate and adaptive immune systems are active even years prior to the onset of RA.
Role of ACPAs and Immune Complexes in the Development of Clinical Disease
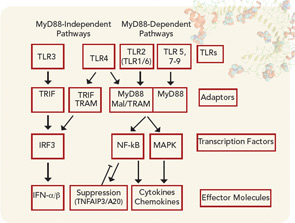
Data have accumulated recently that identify how ACPAs are capable of promoting the development of RA. Circulating immune complexes containing citrullinated fibrinogen have been detected in patients with RA, documenting the ability of citrullinated proteins to form immune complexes.16 Immune complexes formed between citrullinated fibrinogen and ACPAs from patients with RA are capable of activating macrophages to release TNF-α that was mediated not only through Fc receptors but also through TLR4, suggesting that complexes containing citrullinated proteins may be particularly proinflammatory.17 Additionally, these immune complexes containing citrullinated fibrinogen also activated macrophages isolated from the joints of patients with RA.18

