Methotrexate
lyricsaima / Shutterstock.com
MADRID—Tofacitinib (a JAK inhibitor) used with methotrexate (MTX) is not inferior to adalimumab (a TNF inhibitor) in rheumatoid arthritis (RA) patients who’ve had an inadequate response to MTX alone, according to results of a Phase 3B/4 trial presented in a session at the Annual European Congress of Rheumatology (EULAR).
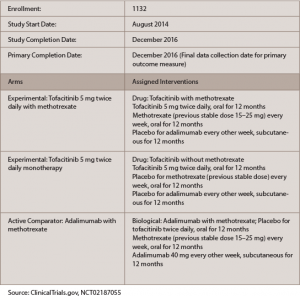
The findings came in a one-year trial, called ORAL Strategy, with three active treatment arms, meant to compare tofacitinib alone with tofacitinib plus MTX, tofacinitib alone with adalimumab plus MTX, and tofacitinib plus MTX with adalimumab plus MTX (see Table 1).1
In the other comparisons, performed using an efficacy threshold derived from a meta-analysis of adalimumab trials, tofacitinib alone landed in a kind of statistical limbo when compared with adalimumab plus MTX: It wasn’t shown to be worse, nor was it shown to be superior. The same was found for tofacitinib alone compared with tofacitinib plus MTX. The main point of the trial was to settle remaining questions from tofacitinib’s Phase 3 trial, which suggested that perhaps tofacitinib alone was better than tofacitinib with MTX—which was found not to be true in this trial—and that tofacitinib with MTX was better than adalimumab.
The Trial
(click for larger image)
Table 1: ORAL Strategy
One thousand one hundred and forty-six patients were randomized: 384 were treated with 5 mg tofacitinib monotherapy orally twice a day, 5 mg tofacitinib twice a day plus MTX or adalimumab plus MTX.
There was no significant difference between the groups, with 46% of the tofacitinib-plus-MTX patients achieving an ACR50 response after six months, compared with 43.8% of adalimumab-plus-MTX patients and 38.3% of the tofacitinib-alone patients. In a subsequent analysis, tofacitinib plus MTX was not found to be superior to adalimumab plus MTX.
“In a group of patients, you can start [tofacitinib] or you can start adalimumab, and you can expect the same result with either,” said Roy Fleischmann, MD, clinical professor of internal medicine at University of Texas Southwestern Medical Center, who presented the results, recently published in The Lancet. He added, “Anyone who interpreted the Phase 3 studies showing that monotherapy [tofacitinib] looks better than the combination of tofacitinib plus MTX should correct their perception.” Monotherapy tofacitinib was found to be “not non-inferior” to tofacitinib plus MTX, landing in a kind of statistical middle ground in which non-inferiority wasn’t demonstrated, but neither was superiority.
The three groups had no major differences in the occurrence of adverse events, except that liver function was better in the tofacinitib-alone group—with at least 12% fewer patients having aspartate transaminase and alanine transaminase at the upper limit of normal or higher, compared with the other treatment groups.