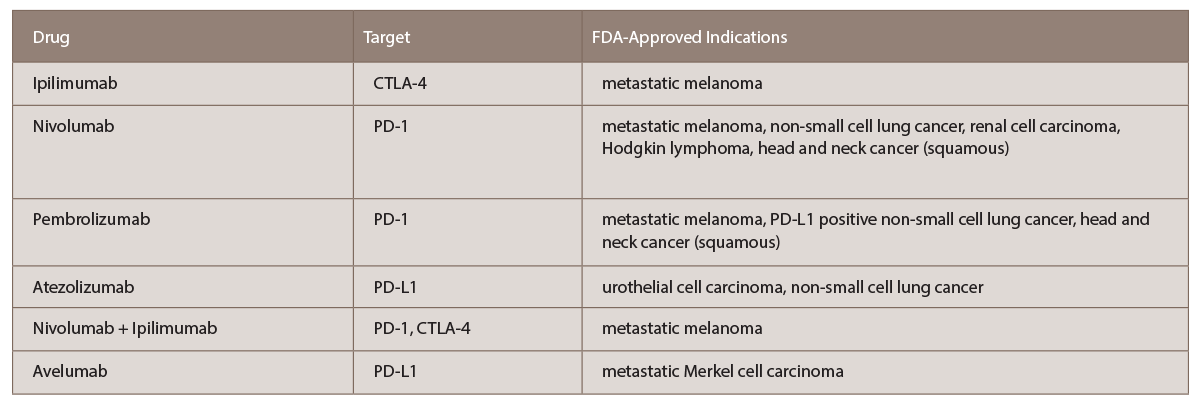
(click for larger image) TABLE 1: List of current FDA-approved immune checkpoint inhibitors with targets and indications (per www.fda.gov).
The idea of using the immune system to fight cancer is more than 120 years old. William B. Coley used a mixture of Streptococcus pyogenes and Bacillus prodigiosus, later referred to as Coley’s toxins, to treat a large inoperable malignant sarcoma, achieving cure.2 The concept of immune system surveillance for the suppression of tumor formation was introduced in 1909 by Paul Ehrlich, not fully gaining acceptance until the early 2000s. Various immunotherapies were tried over the next century, with some success, including interleukin (IL) 2 infusions for treatment of metastatic melanoma and anti-cancer vaccines.
The critical discovery of the cytotoxic T-lymphocyte antigen 4 (CTLA-4) receptor on the surface of T cells occurred in 1986, and 11 years later, antibodies to this receptor were shown to eradicate tumors in mice.1 It was then demonstrated that anti-CTLA-4 antibodies extended life by nearly four months in patients with advanced metastatic melanoma.3 This was the first FDA-approved ICI, subsequently named ipilimumab, an IgG monoclonal antibody to CTLA-4.4
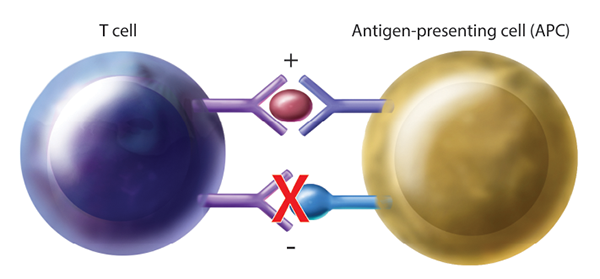
Figure 1: Generalized Mechanism of T Cell Activation Through Immune Checkpoint Blockade. By blocking inhibitory pathways, T cell activation is enhanced, allowing for cancer surveillance and targeted killing. APC=antigen-presenting cell.
Brook Coppola
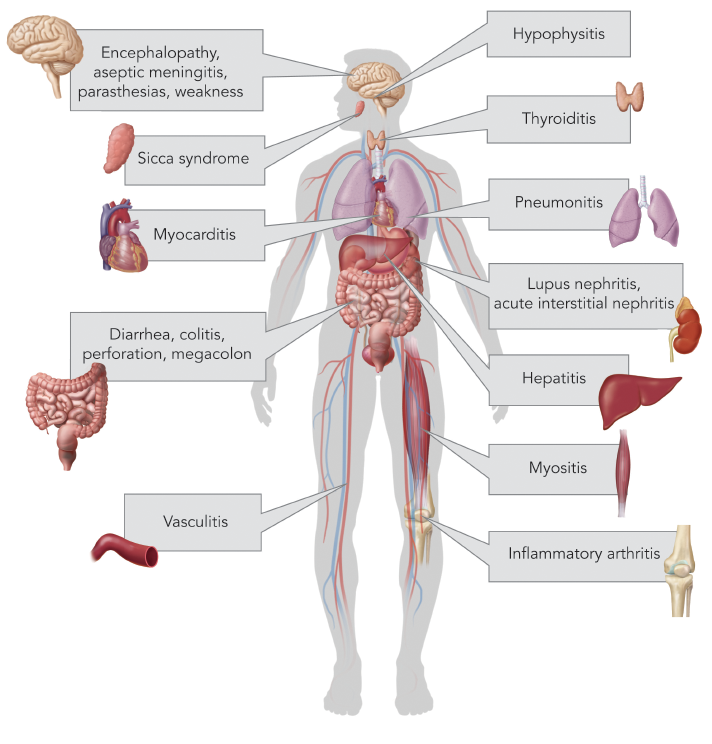
CTLA-4 is a receptor on T cells that, when engaged, inhibits T cell activation. By blocking this inhibitory receptor with CTLA-4 antibodies, T cell activity is upregulated, thus increasing tumor cell targeting and killing (see Figure 1). It is important to note that the activation of T cells is not specific to tumor antigens, thus nonspecific T cell activation may lead to irAEs.5
It is useful to consider the action of abatacept, a fusion protein of the extracellular domain of CTLA-4 and the Fc region of IgG1, which acts in an opposite way of ICIs, by promoting the negative costimulation of T cells.
Monoclonal antibodies to a second major immune checkpoint involved in cancer regulation were in production by the late 2000s. It was noted that programmed cell death receptor ligands (PD-L1), typically expressed on antigen presenting cells, were upregulated in many human cancers, and PD-1 receptors were highly expressed on tumor infiltrating T cells.6 Blocking PD-1 receptors using monoclonal antibodies, including nivolumab and pembrolizumab, also upregulates T cell activation.7 Atezolizumab, directed against the programmed cell death ligand (PD‑L1), has also been approved for treatment of urothelial cell carcinoma and non-small cell lung cancer with similar mechanism.8