The Case
An 18-year-old woman first presented with findings of systemic lupus erythematosus (SLE) including arthritis, fever, photosensitivity, hair loss, oral ulcers, Raynaud’s phenomenon, mild leukopenia, and thrombocytopenia. Antinuclear antibodies and anti-dsDNA were positive, but there was no evidence of nephritis. She was treated with low-dose glucocorticoids and hydroxychloroquine. A few months later, she developed lower-extremity edema, and her urinalysis showed an active urine sediment and proteinuria of 1 g/24 hr. She was treated with prednisone 60 mg/day for presumed proliferative lupus nephritis and is referred to you.
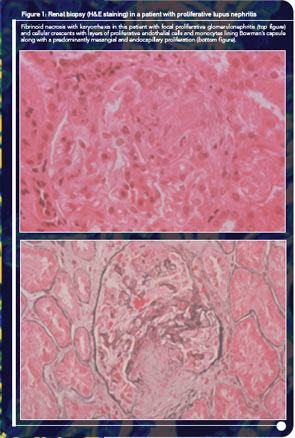
At the time of your evaluation, she is on prednisone 30 mg/day. Urinalysis showed an active sediment with proteinuria of 1.2 g/24 hr. Laboratory values were significant for a hematocrit of 32%, serum creatinine of 1.0 mg/dl, urea 103 mg/dl, and albumin 3.4 mg/dl. Both C3 and C4 titers were low. A renal biopsy showed focal, segmental glomerulonephritis with an activity index of 8 and a chronicity index of 1. On renal biopsy, there were a few areas of fibrinoid necrosis and fibro-epithelial crescents in less than 10% of the renal glomeruli (see Figure 1).
How would you treat this patient? Would you use azathioprine (AZA), mycophenolate mofetil (MMF), or cyclophosphamide (CY)? What is the evidence for your choice?
Although immunosuppressive agents are widely used for the treatment of severe manifestations of SLE, the choice of particular agents is a matter of considerable debate. Among all agents, CY was the first to demonstrate major successes in the treatment of severe disease, especially glomerulonephritis. In the past decade (2000–2010), however, the emergence of MMF for the treatment of nephritis has for some signaled the end of the “cyclophosphamide era.” Should MMF replace CY as the initial treatment of choice for severe disease? With the first biologic agent just recommended for approval by the U.S. Food and Drug Administration, have we entered the “era of biologic therapies” in lupus, with other traditional small molecule agents soon becoming obsolete?
The authors have followed closely the CY and MMF trials both in the United States and in Europe, and we have wrestled with the safety and efficacy issues regarding their use. In this article, we will review the existing data in an effort to provide answers to three fundamental questions regarding immunosuppressive therapy in lupus: Why, When, and How? More importantly, we will argue that the time has come to move our attention away from debating the merits of individual drugs. Rather, we believe that we should focus our efforts on developing a more comprehensive strategy to guide the treatment of lupus. This strategy should both “fit the patient” and achieve timely and long-lasting remission with the lowest possible harm.