Other available treatment options include either nonbiologic DMARDS (hydroxychloroquine, cyclosporine), which most rheumatologists consider less potent, or the newer biologic agents, which clearly alter the host immune response and thus may be harmful to patients with a chronic infection. One class of drugs for which there is a growing safety database is the TNF inhibitors. Anecdotal reports, small retrospective series, and now prospective studies, have all suggested an absence of obvious safety signals, good tolerability, and no obvious influence on underlying HCV infection.15,16 Again, in the absence of biopsy data, ultimate safety cannot be assumed. In addition to these data, a small, single-center trial of etanercept as adjunctive therapy to IFN-a and ribavirin for HCV infection provided evidence of both safety and potential efficacy.17
Based on these observations, a multicenter, randomized, placebo-controlled trial comparing standard antiviral treatment (Peg-IFNa-2b with ribavirin) to the same regimen given in conjunction with another TNF inhibitor (infliximab) is currently underway in order to assess its antiviral efficacy in patients with chronic hepatitis C (ClinicalTrials.gov, NCT00512278). This study will provide pivotal data on the risks and benefits of at least one year of TNF inhibitor exposure in HCV infected patients, as well as critically needed data on the effects of TNF inhibition on liver histology.
Thus, while limited, there appears to be more safety data on the use of TNF inhibitors in the setting of chronic HCV infection than any other class of DMARDs.15 Recently, the ACR also recommended that anti-TNF agents can be used in patients with chronic hepatitis C without evidence of decompensated liver disease.14
Data are even more limited on other biologic agents (abatacept, anakinra) because most studies of new agents are screened to exclude patients with hepatitis. There are some data on the use of rituximab in HCV infection because it has been employed in the management of HCV-associated cryoglobulinemia, again with little evidence of untoward toxicity.18
Should HCV-Infected Patients Be Referred to a Subspecialist and Undergo Biopsy?
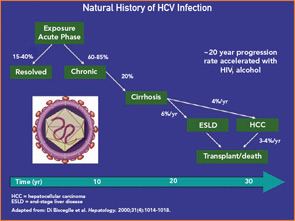
We believe that every patient who is found to be HCV infected should be evaluated by a hepatologist or gastroenterologist with experience in the diagnosis, treatment, and follow-up of patients with chronic hepatitis C. Referral is beneficial to these patients for several reasons. First, it provides the patients the opportunity to get detailed information regarding the nature of their disease, the factors that are associated with an adverse prognosis (alcohol use, obesity, other co-infections, etc.), the available treatment options, and their potential side effects. Second, referral could identify candidates for antiviral therapy that need additional diagnostic work-up, including determination of viral genotype, baseline and post-treatment serum viral loads, imaging of the liver (by ultrasound, CT, etc.), and possibly a baseline liver biopsy. Third, for patients with advanced liver disease (cirrhosis) who are not candidates for antiviral treatment, referral to a tertiary center for evaluation for liver transplantation may be necessary.