PPIs Increase Hip Fracture Risk
By Robyn T. Domsic, MD
Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947-2953.
Abstract
Context: Proton pump inhibitors (PPIs) may interfere with calcium absorption through induction of hypochlorhydria but they also may reduce bone resorption through inhibition of osteoclastic vacuolar proton pumps. Objective: To determine the association between PPI therapy and risk of hip fracture. Design, setting, and patients: A nested case-control study was conducted using the General Practice Research Database (GPRD) (1987–2003), which contains information on patients in the United Kingdom. The study cohort consisted of users of PPI therapy and nonusers of acid suppression drugs who were older than 50. Cases included all patients with an incident hip fracture. Controls were selected using incidence density sampling, matched for gender, index date, year of birth, and both calendar period and duration of up-to-standard follow-up before the index date. For comparison purposes, a similar nested case-control analysis for histamine-2 receptor antagonists was performed. Main outcome measure: The risk of hip fractures associated with PPI use. Results: There were 13,556 hip fracture cases and 135,386 controls. The adjusted odds ratio (AOR) for hip fracture associated with more than one year of PPI therapy was 1.44 (95% confidence interval [CI], 1.30–1.59). The risk of hip fracture was significantly increased among patients prescribed long-term high-dose PPIs (AOR, 2.65; 95% CI, 1.80–3.90; P<.001). The strength of the association increased with increasing duration of PPI therapy (AOR for one year, 1.22 [95% CI, 1.15–1.30]; two years, 1.41 [95% CI, 1.28–1.56]; 3 years, 1.54 [95% CI, 1.37–1.73]; and four years, 1.59 [95% CI, 1.39–1.80]; P<.001 for all comparisons). Conclusion: Long-term PPI therapy, particularly at high doses, is associated with an increased risk of hip fracture.
Commentary
In the United States in 2005, there were more than 95 million prescriptions dispensed for PPIs in 2005, with total sales exceeding $12.8 billion. If we assume one prescription was given per patient, then approximately one-third of Americans were prescribed a PPI. This number is probably an underestimate considering the over-the-counter availability of PPIs. Thus, this study applies to a significant portion of our patients.
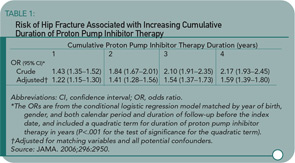

Yang and colleagues used a nested case-control study within the GPRD to examine the association of PPI therapy and hip fractures among individuals 50 or older. There were 192,028 eligible individuals receiving PPIs in the GPRD. Cases were eligible if individuals were followed at least one year in the database prior to suffering an incident hip fracture. Cases were matched up to ten-to-one with non-PPI users by gender, year of birth, index date, and calendar period. The primary exposure of interest was PPI therapy greater than one year before the index date. Steroid use was greater in the PPI users, and this was controlled for in the subsequent multivariate regression model. An additional nested case-control study was performed looking at users of histamine-2 receptor blockers. Shown here are the odds ratio by length of PPI therapy (see Table 1) and PPI or histamine-2 receptor blockers by dose (see Table 2). The results show an increasing risk of hip fracture with increasing duration and higher doses of PPI therapy. Not depicted in these tables is the presence of a stronger association between PPI therapy and hip fracture among men (OR 1.78 [95% CI 1.42–2.22]) than women (OR 1.36 [95% CI 1.22–1.53]), although there was a statistically significant interaction between PPI therapy and gender.