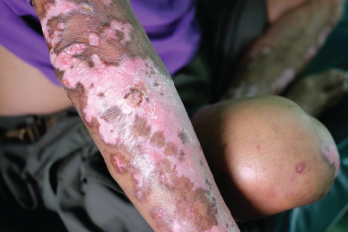
This salt-and-pepper appearance is characteristic of scleroderma.
A yearlong pilot study that evaluated the safety and efficacy of belimumab in a small group of patients with early diffuse systemic sclerosis found no significant difference in the number of adverse events between those treated with the drug and those who received a placebo.
Currently, no drugs are approved specifically for the treatment of systemic sclerosis—also called scleroderma, says Jessica Gordon, MD, MSc, assistant attending physician for the Department of Rheumatology at the Hospital for Special Surgery (HSS) in New York City and first author of the study published recently in Arthritis & Rheumatology. A human monoclonal antibody that inhibits B cell activating factor, belimumab (Benlysta)—an FDA-approved treatment for lupus—was chosen as a candidate for the pilot study because of the important role B cells play in the pathogenesis of systemic sclerosis.
“There are similarities between scleroderma and lupus, including a significant role for B cells, so in this trial we investigated whether we could reappropriate a treatment that was already approved for lupus,” explains Dr. Gordon.
Background
Scleroderma is a rare autoimmune connective tissue disease that causes thickening of the skin and leads to complications for the lungs, heart, kidney and other organs, as well as vascular dysfunction, as often evidenced early on by Raynaud’s phenomenon, which causes numbing and coldness in the hands and feet. Patients may experience trouble breathing, cardiac arrhythmia, gastrointestinal difficulties and joint pain.

Dr. Gordon
The disease is also highly heterogeneous: Organ system involvement and disease manifestation tend to differ from one patient to the next, says Dr. Gordon.
“One patient may have very severe skin or musculoskeletal disease,” she says. “Another patient may have very mild skin disease but could have severe lung disease.”
The Study
The industry-sponsored study involved 20 patients in a randomized, double-blind, placebo-controlled trial conducted by an investigator at a single center at HSS. All participants were 18 years or older, met ACR preliminary criteria for systemic sclerosis and had early diffuse systemic sclerosis of three years’ or shorter duration.
The primary objective was to assess the safety and tolerability of belimumab in patients by comparing the number of adverse events between the belimumab and placebo groups. The primary efficacy endpoint was the difference in skin thickness as assessed by the modified Rodnan skin score (MRSS) after 52 weeks.
Secondary efficacy endpoints were evaluated with the use of surveys and questionnaires. Skin biopsies and serum B-lymphocyte stimulator levels also were assessed.


