Despite a generation of advances in molecular biology, a huge gap exists between the Sjögren’s syndrome (SS) patient’s description of their symptoms and the objective findings. Current issues include:
- Many SS patients are misclassified as either rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE), even within rheumatology clinics.
- Frequently, the sickest SS patients with extraglandular manifestations, including lung, kidney and neurologic system, may be directed to other specialty clinics (e.g., Pulmonary, Renal, Hematology) where the illness is mislabeled as SLE or idiopathic.
- As a result, our SS clinical trials are biased toward SS patients with only sicca complaints and fatigue—the manifestations least likely to show benefits in trials studying biologics or other novel immunomodulation agents.
- As a result of these factors (see Table 1, right), there has been growing discouragement among both patients and rheumatologists about the likelihood of discovering novel therapies for SS.
Steps for the future: First, improvement in identifying SS patients will require relabeling the interpretation of the ANA (the gold standard is immune fluorescent antibody method or IFA) as suggestive of SLE or SS.
- A reflex antibody to SS-A/B should be performed if ANA by IFA is positive.
- If the ANA is negative by enzyme-linked immune assay (ELISA), then a reflex antibody to SS-A/B should still be performed, since the ANA by ELISA test may fail to identify the SS-A/B antibody.
Second, we have become prisoner to the paradigm of symptoms and acute-phase reactants, such as erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP).
- This follows from a decade of understanding T cell-mediated mechanisms of antigen-dependent-driven processes.
However, this model clearly does not help in judging activity of central nervous system (CNS) processes in known autoimmune disorders such as multiple sclerosis (MS). We must understand how neural-mediated transmitters play a role in the CNS.
Third, we must develop an understanding of the “neuro-immune” interface that governs the cortical sensation of dryness or pain.
- Our failures in better managing neuropathic pain become very evident when managing our patients with SS.
- Thus, a better collaboration between rheumatology and other medical specialties, such as neurology, and pain medicine for clinical trials should improve the outlook for the next decade’s therapy.
In a previous issue of The Rheumatologist (November 2013), we described a model of “phantom pain” and how it might play a role in the difference between patients’ symptoms and objective findings.
Background
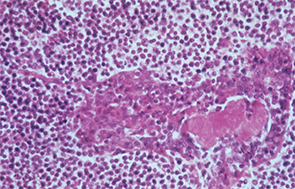
In this patient with Sjögren’s syndrome, most of the parotid gland parenchyma has been replaced by a diffuse collection of lymphocytes, which sometimes leads to an erroneous histologic diagnosis of malignant lymphoma. The lumen of the salivary ductule in the center of the field is occluded by an eosinophilic deposit of inspissated or thickened secretion; the duct lining cells are markedly hyperplastic (epimyoepithelial islands).
Image Credit: Rheumatology image library