Two studies have been conducted in recent years with implantable vagus nerve stimulation devices as a possible treatment for RA. These studies demonstrate improvements in systemic inflammation & disease activity.
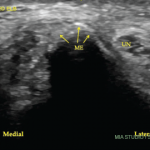
Of 30 patients enrolled in the study, 27 completed the week 12 visit. Patients used the device on 94% of the study days, and this measurement of compliance has high validity given that compliance was automatically tracked by the devices. A significant reduction was seen in DAS28-CRP after week 1 and this score remained significantly reduced at week 12. Some 37% of patients reached DAS28-CRP of 3.2 or less, which indicates low disease activity, and 23% of patients reached DAS28-CRP of less than 2.6, which indicates remission, at week 12. In addition, 53% of patients reached ACR20, 33% reached ACR50 and 17% reached ACR70. With respect to imaging, the mean change in ultrasound synovitis and tenosynovitis scores were statistically significant and, for MRI, reductions in synovitis, osteitis, bone erosion and cartilage loss scores at week 12 compared to baseline were seen, although these were not statistically significant.6
Lead author Sara Marsal, MD, associate professor of rheumatology and head of rheumatology, Vall d’Hebron University Hospital, Autonomous University of Barcelona, Spain, summarizes the key clinical takeaways from this study. “The proof-of-concept clinical data show meaningful clinical benefit for the majority of participants, as assessed by the usual clinical endpoints (ACR20/50/70 and DAS28-CRP) and a very favorable risk/benefit profile,” Dr. Marsal explains. “Seeing low disease activity and remission for some patients was encouraging. Imaging of the joints with ultrasound, which is common practice in Europe, reinforced the benefit.”
In discussing the study results, Dr. Baker notes that patient compliance with the device was high, as were patient satisfaction scores. He explains that because the earpieces were custom-molded for each patient and the treatment is designed to be imperceptible, it is a comfortable experience for the patient. In addition, very few adverse events occurred; in fact, the only device-related event was a superficial skin abrasion in one patient. Given that customization of devices is quick and inexpensive, it would be expected that this would be possible even if and when the devices are available for widespread use. Such customization improves comfort for patients and helps ensure proper functioning.
It is important to understand potential limitations of this study, including some of the trial’s exclusion criteria. Patients with a history of previously implanted electrically active medical devices, such as cardiac pacemakers or automatic implantable cardioverter defibrillators, were excluded from participation. Because this was the first open-label pilot study of this noninvasive vagus nerve stimulation device, Dr. Genovese explains, it made sense to exclude these patients out of an abundance of caution. However, it would be reasonable to think the stimulation device could be safely used in these patients in the future and that such patients may not necessarily be excluded from larger future trials but preliminary studies in this population would need to demonstrate no cross interference.
In addition, the patient population in this study was predominantly naive to biologic DMARDs, thus it is not known if the benefits of treatment with the device would be seen in patients who are refractory to biologic DMARD or targeted synthetic DMARD treatment. Finally, vagus nerve stimulation was not combined with treatment with biologic or targeted synthetic DMARDs; thus, it is unknown if a potential additive effect of these treatments could occur when used together.
Dr. Genovese provides helpful insight, stating, “Although limited conclusions can be reached from open label studies and those with relatively small numbers of patients, one can’t help but be enthusiastic about the possibility of noninvasive vagus nerve stimulation as an adjunctive treatment in helping reduce some of the symptoms associated with RA.”
John Miller, MD, an instructor of medicine in the Johns Hopkins Arthritis Center, notes, “While there are inherent limitations in a pilot study like this, the results are compelling and warrant further examination in a randomized process, particularly with an adequate placebo group.
“If these data are replicated in larger studies,” he adds, “it will also be important to determine which subsets of patients respond best to therapy. Other teams have shown similar results with vagal nerve stimulation, though there was suggestion that response may be related to underlying disease activity.”7