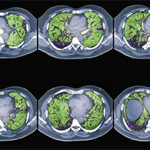
Diagnosis typically involves assessing pulmonary function and examining high-resolution computed tomography (HRCT) scans. Bronchoscopy also may be a useful diagnostic tool, along with bronchoalveolar lavage to assess for infection and alveolar hemorrhage, Dr. Fischer said. Transbronchial biopsy may not add much useful information, he added.
It’s important for pulmonologists to get rheumatologists involved in diagnosis and treatment for these patients, Dr. Fischer said. ILD has a “rheumatologic flavor,” he said, with some patients presenting with specific autoantibodies and histopathologic features. With connective tissue–associated ILD, however, there often is a lack of extrathoracic features, he said.
How should these conditions be treated? For ILD in systemic sclerosis (SSc-ILD), the rheumatologist must first ask whom to treat, when to treat, and what therapy to try, said Richard Silver, MD, professor of medicine and pediatrics at the Medical University of South Carolina in Charleston.
Severity of SSc-ILD is greater in African Americans, males, and patients over age 70, Dr. Silver said. Risk factors include patients who are positive for the Scl-70 or U11/U12 RNP antibody and patients with severe esophageal disease, he said. “We have to look at patients phenotypically, seriologically, and, in the future, genotypically as well, to identify patients who are at the highest risk” of developing ILD, he added.
In the future, rheumatologists may be able to identify who is at higher risk for ILD and target those people for more aggressive therapy, Dr. Silver said. Serum biomarkers for SSc-ILD include SP-D and KL-6. Patients who had both SSc and alveolitis by bronchoalveolar lavage or ground glass on HRCT chest scan had higher serum levels of both SP-D and KL-6, a potentially prognostic factor in the decline of lung function in these patients, he added.
Targeting Treatment
When should rheumatologists observe and wait to treat patients, and when should they intervene immediately? HRCT results showing the extent of ground glass opacity and forced vital capacity (FVC) levels are two key determinants, Dr. Silver noted. For example, if FVC is greater than or equal to 70% predicted, it may be best to observe and delay treatment. If FVC is less than 70% and lung function is declining, treatment may be warranted.
Cyclophosphamide is an effective treatment, but toxicity concerns make long-term use problematic, Dr. Silver said. Other treatments now in clinical trials are mycophenolate mofetil, imatinib mesylate, and rituximab. In the future, treatments like immunosuppressive therapy, antifibrotic therapy, or cell-based therapy with hematopoietic or mesenchymal stem cells may help these patients, he said. Biomarker research may soon offer more useful clues for treatment options, Dr. Silver said. “At this point, it is the art and not the science of medications,” he concluded.