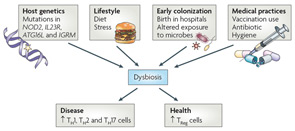
Figure 4
Proposed causes of dysbiosis of the microbiota. The composition of the microbiota can shape a healthy immune response or predispose to disease. Many factors can contribute to dysbiosis, including host genetics, lifestyle, exposure to microorganisms, and medical practices. Host genetics can potentially influence dysbiosis in many ways. An individual with mutations in genes involved in immune regulatory mechanisms or proinflammatory pathways could lead to unrestrained inflammation in the intestine. It is possible that inflammation alone influences the composition of the microbiota, skewing it in favor of pathobionts. Alternatively, a host could “select” or exclude the colonization of particular organisms. This selection can be either active (as would be the case of an organism recognizing a particular receptor on the host) or passive (the host environment is more conducive to fostering the growth of select organisms). Selection of pathobionts by the host could tip the balance in favor of inflammation. Diet and stress also have the potential to influence the microbiota103. Birth in the sterile environment of hospitals can protect from exposure to dangerous pathogens, but can also prevent early exposure to health-promoting bacteria. Overuse of vaccination and antibiotics, which do not distinguish between pathogenic or symbiotic microorganisms, could adversely alter the microbiota.
Abbreviations: ATG16L, autophagy-related gene 16-like; IGRM, immunity-related GTPase family, M; IL23R, interleukin-23 receptor; NOD2, nucleotide-binding oligomerization domain 2; Th, T helper; TReg, regulatory T.
Source: Nat Rev Immunol. 2009;9:313-323. Reproduced with permission of Nature Pub. Group.
The intricate mechanism by which the gut microbiome may modulate systemic disease was recently demonstrated in mouse models of arthritis. When raised under germ-free conditions, both the IL-1 receptor antagonist-knockout (IL1rn-/-) mice and the K/BxN T-cell receptor transgenic model are resistant to inflammatory arthritis.20,21 The introduction of a single gut commensal bacterium (Lactobacillus and SFB, respectively) is sufficient for the development of joint disease. Interestingly, in both models, it is the overactivation of Th17 cells and the dampening of Treg function (via dendritic cells and TLR signaling) that drive systemic autoimmune disease. This model of dysbiosis, leading to local immune imbalance in favor of a proinflammatory Th17-driven state to ultimately promote distal autoimmunity, is not unique to RA. Experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis, is abrogated in germ-free conditions, and also with antibiotic or probiotic treatment.22 Tolerogenic Tregs are responsible for these protective responses. In contrast, EAE susceptibility and severity are enhanced by the administration of bacteria such as Porphyromonas gingivalis and SFB.
Conclusions
The role of microbiome in autoimmunity appears to be far-reaching and important. Clear disease examples of the influence of gut–joint axis include spondyloarthropathies (i.e., reactive arthritis and inflammatory bowel disease–related arthritis), jejunoileal bypass arthritis, and Whipple’s disease.