Just as a rising tide lifts all boats, the Rheumatology Informatics System for Effectiveness (RISE) registry lifts all rheumatology research. Developed by the ACR, RISE initially arose from federal reporting requirements.
Although the initial role of RISE was to help clinicians navigate the changing payment landscape and track quality of care, it has also become a vast repository of unique data on millions of rheumatology patients from across the U.S.
RISE at a Glance
“We’re proud to say the RISE registry is the first and largest national EHR-enabled rheumatology registry in the country, with more than 30% of U.S. rheumatologists participating,” says Rachel Myslinski, vice president of practice, advocacy & quality for the ACR.
The RISE data set is a unique treasure trove, with data on more than 1.5 million patients representing about 17 million encounters. “The information in the RISE registry is real-world data collected during the routine course of clinical care,” says Tracy Johansson, the ACR’s director of registry analytics. “In short, it represents what clinicians are actually doing in their practices.”
How to Access the Data for Research
Researchers interested in using RISE data for research can submit requests through the ACR’s online request form. All requests are reviewed by ACR staff and the ACR’s Research and Publications Subcommittee, a group of volunteers familiar with RISE data and experienced in using EHR data for research.
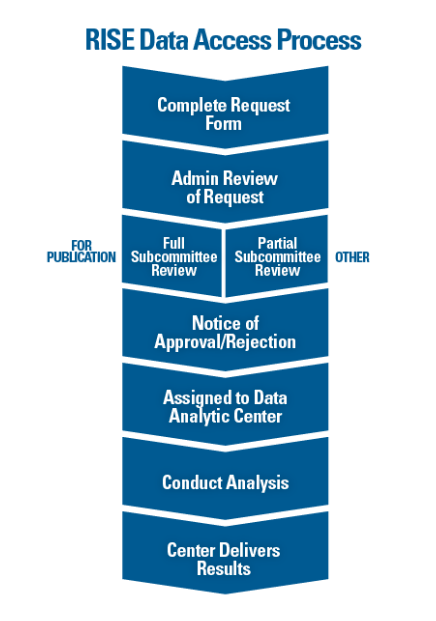 Katherine Liao, MD, MPH, chair of the ACR’s Research and Publications Subcommittee, says the review process is focused on feasibility (ensuring the questions posed can be answered by RISE data) and preventing overlap of projects. However, subcommittee members can provide valuable feedback. “Individuals who sit on the subcommittee are all funded investigators. Each person knows how to think deeply about pertinent research questions, and we all have our fingers on the pulse of the rheumatology community.”
Katherine Liao, MD, MPH, chair of the ACR’s Research and Publications Subcommittee, says the review process is focused on feasibility (ensuring the questions posed can be answered by RISE data) and preventing overlap of projects. However, subcommittee members can provide valuable feedback. “Individuals who sit on the subcommittee are all funded investigators. Each person knows how to think deeply about pertinent research questions, and we all have our fingers on the pulse of the rheumatology community.”
The review process is iterative, allowing requestors to make updates to their proposal as they see fit based on the feedback they receive at each stage. Once a request is approved, the project is assigned to one of three RISE data analytic centers (DACs)—University of California, San Francisco (UCSF), University of Alabama, Birmingham, and Duke Clinical Research Institute—each of which is led by a rheumatology expert.
To be clear, researchers do not have direct access to the data. “The database is not set up so an investigator can actually touch the data,” says Dr. Liao. “The data must be cleaned and maintained, and we must be able to ensure confidentiality. This means that requestors work very closely with the team at the assigned DAC.”